Product calcium cyanamide

Manufacture
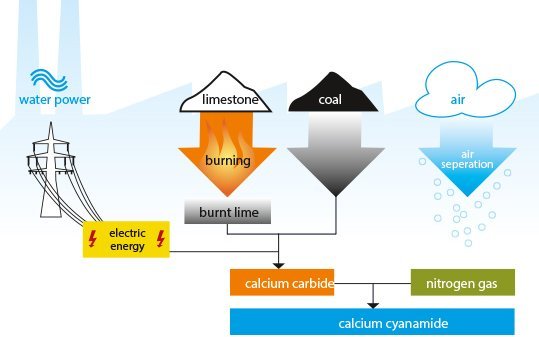
Calcium cyanamide manufacture
Limestone, coal and atmospheric nitrogen are the natural raw materials from which calcium cyanamide is manufactured. Regular investment and constant improvement of the production process ensures a modern, efficient and environmentally friendly manufacture of the specialty fertilizer.
Calcium cyanamide is manufactured in three stages:
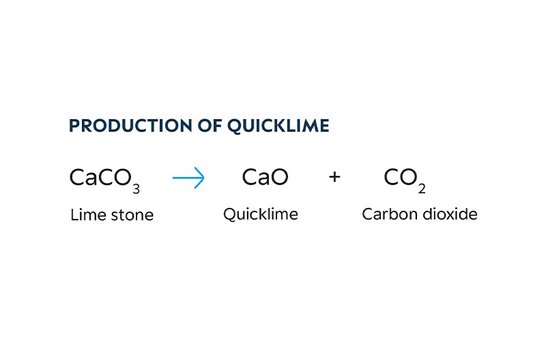
- Stage one: Manufacture of burnt lime (also known as quick lime)
Limestone (CaCO3), mined from natural deposits, is crushed and then processed in coke or oil-fired furnaces to form burnt lime. - Stage two: Manufacture of carbide
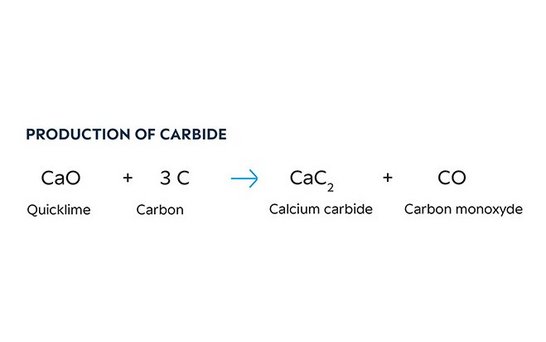
The burnt lime obtained as a result of this process is fed into carbide furnaces together with coke and anthracite, which provide a supply of carbon. Calcium carbide is produced in these ovens at temperatures above 2,000°C, with electrical energy generating the heat required. The carbon monoxide generated during this manufacturing process is collected and used as a starting material for further chemical reactions. - Stage three: Manufacture of calcium cyanamide
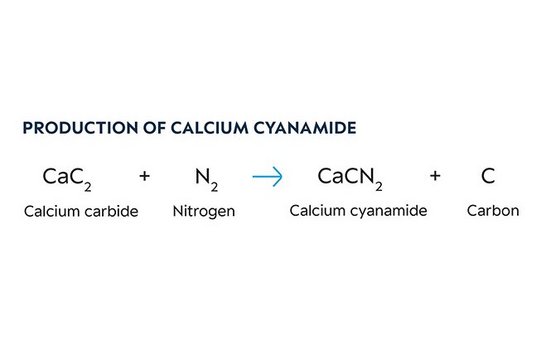
In the last stage of the synthesis nitrogen gas is passed through finely-ground calcium carbide which has a temperature of approx. 1,100°C. This chemically binds the nitrogen, resulting in calcium cyanamide. The carbon released during this reaction gives the calcium cyanamide its black color. The nitrogen gas necessary is recovered in a proprietary air separation plant using the Linde method.
The so-called technical calcium cyanamide coming from the rotary kilns is further processed for use in agriculture and horticulture. The ground-calcium cyanamide is produced by finely grinding the product. PERLKA® (= pearled calcium cyanamide) is produced from the ground product by granulation, and adjusted to the defined grain size by sieving.
Types
Perlka® Nitrogenous calcium cyanamide 19.8
PFC 1(C)(l)(a)(i): Straight solid inorganic macronutrient fertiliser
| Nutrient composition Perlka® |
|---|
| 19.8 % Total nitrogen (N) |
| 1.8 % Nitrate nitrogen |
| 1.3 % Urea nitrogen |
| 15 % Cyanamide nitrogen |
| 48 % Total calcium oxide (CaO) |
| 13 % Water-soluble calcium oxide (CaO) |
| 1.5 % Total magnesium oxide (MgO) |
| Physical properties | PERLKA® |
|---|---|
| appearance and Composition | Gray-black granulate |
| Pouring density | 1000kg/m³ |
| Grain size | 90 % of the product passes through a sieve with a mesh size of 3.5 mm (macro/standard) or 1.7 mm (micro) |
| Packaging | PERLKA® |
|---|---|
| PSE sack, palletized | 25 and 50 kg |
| big pack | 600 kg |
| unpackaged | loose |
| Small packets | 5 and 10 kg |
Quality
Secure product quality
Quality is critically important in the production of calcium cyanamide. It begins right from the selection of the natural raw materials. Only materials which have a specific minimum degree of purity are used for production. Of course, environmentally friendly production is also a part of the quality of the product. This is why the production process for calcium cyanamide has been and is being constantly refined, the aim being to save valuable resources and energy and avoid waste and emissions. As an example, the ammonia emission of the PERLKA® plant was cut by more than 80% in the period 1989 through 2000.
Miscibility
PERLKA® as a mixing component
The systematic mixing of fertilizers ensures that the nutrients required for balanced plant nutrition can be applied in one operation. PERLKA® calcium cyanamide can also be used as a mixing component.
In this case you should note the following points, however:
- Do not mix it with fertilizers containing ammonium nitrate! This could set off chemical reactions that could make the mixture greasy and result in ammonia loss.
- All mixtures should always be stored dry! Cover loose goods with a tarpaulin.
- Mixtures with hygroscopic-acting fertilizers should be spread as quickly as possible to avoid lumping.
- To ensure an even distribution when spreading the mixture make sure that the mixture components have roughly the same grain sizes and specific gravities.
The following table shows the miscibility of PERLKA® with selected mineral fertilizers:
| Mixture component | Miscibility |
|---|---|
| Ammonium sulphate | + |
| Urea | + |
| Kainite | + |
| Potassium sulphate | + |
| Kieserite | + |
| Potassium chloride (KCl), | + |
| Rock phosphate | + |
| DAP (diammonium phosphate) | + |
| Excello | + |
| Calcium nitrate | o |
| Triple-superphosphate | - |
| MAP (monoammonium phosphate) | - |
| Ammonium sulphate nitrate | - |
| ENTEC | - |
| CAN calcium ammonium nitrate | - |
Key:
+ miscible and storable
o miscible but not storable
- not miscible
Storage
Notes on storage
- The storage area must be carefully cleaned before equipping with calcium cyanamide and PERLKA®, and this is especially the case with loose goods
- Calcium cyanamide must be stored dry and protected from damp
- Do not store it together with highly flammable and combustible materials and substances
- Store it separately away from fertilizers containing nitrates, and away from substances that are acid and alkaline reactive
- Calcium cyanamide PERLKA® may only be stored together with ammonium- and ammonium nitrate-containing fertilizers when it is adequately separated from them. Adequately separated means:
- A minimum distance of 5 m when stored loose outdoors
- A minimum distance of 2.50 m when stored loose in a storage room
- A minimum distance of 1 m for packaged products in a storage room
(non-reactive substances can be stored in between)
- With loose calcium cyanamide PERLKA® there are no material-related storage problems since the product does not corrode wood, concrete, plastics or steel.
- When storing in flat stores the usual principles for loose storage of mineral fertilizers should be observed (cover with PE film)
- Storage in tower silos is straightforward; as long as damp is prevented from getting in there should be no bridging and caking
- Transport and interim storage in sloped-bottom containers is also straightforward.
Safety Information
Please note the following safety instructions when working with calcium cyanamide:
- Keep out of the reach of children!
- Do not consume any alcoholic drinks before, during and after working with calcium cyanamide
- Do not eat, drink or smoke when using it
- Wash hands before breaks and immediately after handling the product
- Shower or bathe at the end of work
- Avoid breathing in dust! If there are large amounts of dust use a half mask with a particle filter (DIN EN 149 FFP2)
- Avoid contact with the eyes and avoid skin contact! (wear suitable, long-sleeved protective clothing and protective goggles)
- If the product gets in the eyes immediately rinse them with lots of water for several minutes (seek medical attention)
- Prevent animals from consuming calcium cyanamide
Classification of calcium cyanamide / PERLKA®:
- Nonhazardous goods in terms of transport regulations
- Water hazard class 2
- Signal word: Danger
- GHS hazard statements: H302, H315, H317, H318, H335, H412, P261, P280, P301+P312, P302 + P352, P304 + P340, P305 + P351 + P338
Notes on fire protection:
- PERLKA® calcium cyanamide are neither self-combustible nor incendiary
- Intense heat exposure can result in decomposition with ammonia and nitrous gases formed as a result
- If fire breaks out at a storage facility or in the storage building wear a self-contained breathing apparatus and chemical protective suit
- Suitable extinguishing agent: Extinguishing powder, water spray, dry sand.
In the event of fire or decomposition please contact our Trostberg plant immediately. Phone: +49 8621 86-2776
Always follow the instructions in our Safety Data Sheet!
You can also obtain these from us at:
Alzchem Trostberg GmbH
Agriculture business unit
Postbox 1262
83303 Trostberg, Germany
Phone: +49 8621 86-0
Fax: +49 8621 86-2446
Email: perlka@alzchem.com
History
Calcium cyanamide – an agricultural production resource with a long tradition and a bright future
Calcium cyanamide originated in 1895 when Adolph Frank and Nikodem Caro discovered that gaseous nitrogen is taken up by alkaline earth carbides.
The nitrogen obtained through air separation binds with calcium carbide at temperatures around 1,100°C, to form calcium cyanamide. Calcium cyanamide was the first mineral fertilizer that enabled atmospheric nitrogen to be used for plant nutrition.
At first it was used as a nitrogen and lime fertilizer, but it was soon discovered that it had a positive secondary effect on the health of soils and plants.
This is because it converts in the soil over a longer period of time, so its nitrogen is protected to a large extent against nitrate leaching and also against emission as nitrogen oxide, particularly beneficial from the point of view of environmental protection. Calcium cyanamide has been a reliable partner in agriculture and horticulture for more than 100 years, and can be relied upon in the future as well.